Prescient Surgical, Inc., a medical device innovator based in San Carlos,
California just
won FDA clearance for commercializing its novel CleanCision wound retraction
and protection system.
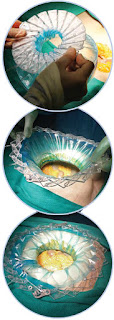
This system is one of its kind, advanced surgical protection system designed by surgeons and infection control specialists. It is used by surgeons during abdominal surgery to retract the surgical incision, providing easy access to the surgical site while protecting the edges with an impermeable barrier thereby reducing the chances of wound infection.
The device is placed in the wound and
pulled apart, to open the radial fan like expanding system that is suitable for
incisions ranging from 7-16 cm. The wound edges are continuously irrigated
by an irrigant fluid of surgeon’s choice
with the help of gravitational force from an external fluid bag and the excess is
drained via a suction connected to the device. The impermeable inner layer of
retractor prevents the wound contamination by fluids in the operative field.
Studies have
shown a 61% reduction in rates of SSI using this device. The product is
initially promoted for abdominal surgery and particularly colorectal surgery,
where the risk, frequency and severity of surgical site infection is high, and
the need is acute. But, it is useful in other specialties like Oncology and
Ob/Gyn also.
The clearance could not have
come at better time because "Hospitals are increasingly focusing on
infection control to improve patient care as a primary goal. Prescient's
success with the CleanCision device bodes well for providers driven towards the
'triple aim' of quality of care, patient satisfaction, and reduced costs,"
says Brant Heise, Managing Director at Summation Health Ventures.
The 2016 Surgical Site Infection
updated guidelines, published by the American College of Surgeons and Surgical
Infection Society, report that the risk of SSI is generally 2 to 5 percent with
an estimated 160,000 to 300,000 SSIs occurring annually in the U.S.2 The
rate of SSI can be as high as 15 to 30 percent in certain high-risk,
clean-contaminated, and contaminated procedures such as colorectal surgery.3 National
focus on SSI is increasing due to public reporting of surgical site infection
rates and significant financial penalties imposed on hospitals, brought about
by the Centers for Medicare and Medicaid Service's (CMS) Hospital-Acquired
Condition Reduction Program.